Mikrogen Diagnostik recomLine Toxoplasma IgG (Avidity) Lateral Strip Test Kit (5972)
$577.00
SKU: 5972
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomLine Toxoplasma IgG (Avidity) Lateral Strip Test Kit (5972)
Description Strip-Immunoassay with antigens produced by recombinant techniques for the detection of IgG, IgM and IgA antibodies against Toxoplasma gondii
Product is intended for research use.
Product is intended for research use.
Target Human Toxoplasma gondii IgG
Species Reactivity Toxoplasma
Assay Type Indirect Sandwich ELISA
Applications Lateral Strip Assay
Associated Products Toxoplasma gondii Antigen (BA110VS)
recomLine Toxoplasma IgM (IgA) Lateral Strip Test Kit (5973)
Toxoplasma gondii IgG Control (C110G)
Toxoplasma gondii IgM Control (C110M)
Toxoplasma gondii IgG ELISA Kit (ESR110G)
Toxoplasma gondii IgM ELISA Kit (ESR110M)
Toxoplasma gondii Avidity Control IgG (BR110AVID)
recomLine Toxoplasma IgM (IgA) Lateral Strip Test Kit (5973)
Toxoplasma gondii IgG Control (C110G)
Toxoplasma gondii IgM Control (C110M)
Toxoplasma gondii IgG ELISA Kit (ESR110G)
Toxoplasma gondii IgM ELISA Kit (ESR110M)
Toxoplasma gondii Avidity Control IgG (BR110AVID)
Properties
Background Toxoplasma gondii shows the highest incidence of any human pathogenic parasite in central Europe. As a rule, the course of the infection is asymptomatic or mild and confers lifelong immunity on immunocompetent persons. Initial contact with the pathogen during pregnancy can result of transmission of the pathogens to the foetus, resulting in severe damage to it.The main task of routine diagnostics is therefore a preventive testing before or during pregnancy. Due to the frequently subclinical course and the mostly uncharacteristic clinical picture, a clinical diagnosis is impossible without laboratory tests and is mainly based on serological methods with its main interest to differentiate acute infections from past infections. It is well known that Toxoplasma specific IgM antibodies can persist for month or even years after initial contact, so a positive IgM finding is not sufficient for the diagnosis of an acute toxoplasmosis. Much more of importance is to determine the avidity of Toxoplasma specific IgG antibodies.The recomLine Toxoplasma is worldwide the only test system which meets the requirements for the special diagnosis of toxoplasmosis especially for pregnancy. The optimized combination of using recombinant antigens for IgG and IgM and also 4 different phase specific avidity antigens is unique on the market for the determination of the status of a Toxoplasma infection.
Sample Type Serum, plasma, whole blood
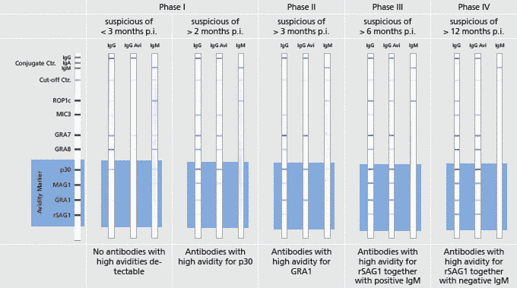
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| TMB Substrate | 40 mL |
| Milk Powder | 5 g |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Test Strips | 2 Vials x 10 Each |
| Anti-Human IgG Conjugate | 500 uL |
Specificity Information
Target ID Human Toxoplasma gondii IgG
Research Areas Infectious Disease
Application Images







Description Strip layout.

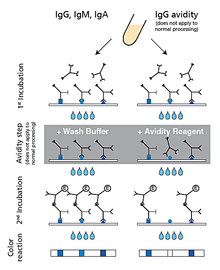
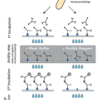
Description Test principle and procedure.
1. 1st Incubation. One (two*) strip loaded with Toxoplasma antigens is incubated with diluted serum or plasma in a dish for 1 hour.
2. Wash 3 (1*) times
3. Avidity step* (IgG only - does not apply to normal processing). Wash buffer is added to one strip and the dissolved avidity reagent is added to the other strip, followed by a 3 minute incubation. Low avidity antibodies will be “washed off” by the avidity reagent.
4. Wash 3 times
5. 2nd Incubation
6. Peroxidase conjugated anti-human antibodies (IgG, IgM or IgA specific) (IgG specific only*) are added. Incubate for 45 minutes.
7. Wash 3 times
8. Color reaction. 8 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies. (By direct comparison of the intensity of the corresponding bands on the two strips, the avidity of individual anti-Toxoplasma IgG antibodies can be determined.*)
(* processing of avidity determination)
1. 1st Incubation. One (two*) strip loaded with Toxoplasma antigens is incubated with diluted serum or plasma in a dish for 1 hour.
2. Wash 3 (1*) times
3. Avidity step* (IgG only - does not apply to normal processing). Wash buffer is added to one strip and the dissolved avidity reagent is added to the other strip, followed by a 3 minute incubation. Low avidity antibodies will be “washed off” by the avidity reagent.
4. Wash 3 times
5. 2nd Incubation
6. Peroxidase conjugated anti-human antibodies (IgG, IgM or IgA specific) (IgG specific only*) are added. Incubate for 45 minutes.
7. Wash 3 times
8. Color reaction. 8 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies. (By direct comparison of the intensity of the corresponding bands on the two strips, the avidity of individual anti-Toxoplasma IgG antibodies can be determined.*)
(* processing of avidity determination)

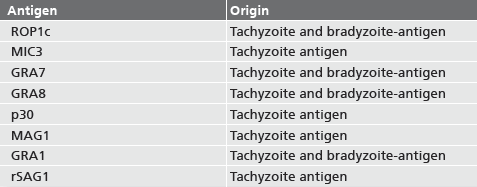
Description Related recombinant Toxoplasma antigens

Description Example data.
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet