Toxoplasma gondii IgG ELISA Kit (ESR110G)
$362.00
SKU: ESR110G
Categories: Infectious Disease Test Kits and Positive Controls, VirionSerion ELISA
Overview
Product Name Toxoplasma gondii IgG ELISA Kit (ESR110G)
Description SERION ELISA classic Toxoplasma gondii IgG/IgM are quantitative and qualitative tests for detection of human antibodies in serum or plasma against Toxoplasma gondii.
Product is intended for research use.
NOTE: Pretreatment of samples with RF-Absorbent (Z200) is recommended for use with IgM ELISA kits to eliminate presence of sample rheumatoid factors and possible false negative results.
Click here to see Rheumatoid Factor (Rf)-Absorbent (Z200) for IgM-Antibody-Detection
Please refer to the kit protocol below for additional information.
Product is intended for research use.
NOTE: Pretreatment of samples with RF-Absorbent (Z200) is recommended for use with IgM ELISA kits to eliminate presence of sample rheumatoid factors and possible false negative results.
Click here to see Rheumatoid Factor (Rf)-Absorbent (Z200) for IgM-Antibody-Detection
Please refer to the kit protocol below for additional information.
Target Human Toxoplasma gondii IgG
Species Reactivity Toxoplasma gondii
Assay Type Indirect ELISA
Applications ELISA
Associated Products Toxoplasma gondii Antigen (BA110VS)
recomLine Toxoplasma IgG (Avidity) Lateral Strip Test Kit (5972)
recomLine Toxoplasma IgM (IgA) Lateral Strip Test Kit (5973)
Toxoplasma gondii IgG Control (C110G)
Toxoplasma gondii IgM Control (C110M)
Toxoplasma gondii IgM ELISA Kit (ESR110M)
Toxoplasma gondii Avidity Control IgG (BR110AVID)
recomLine Toxoplasma IgG (Avidity) Lateral Strip Test Kit (5972)
recomLine Toxoplasma IgM (IgA) Lateral Strip Test Kit (5973)
Toxoplasma gondii IgG Control (C110G)
Toxoplasma gondii IgM Control (C110M)
Toxoplasma gondii IgM ELISA Kit (ESR110M)
Toxoplasma gondii Avidity Control IgG (BR110AVID)
Properties
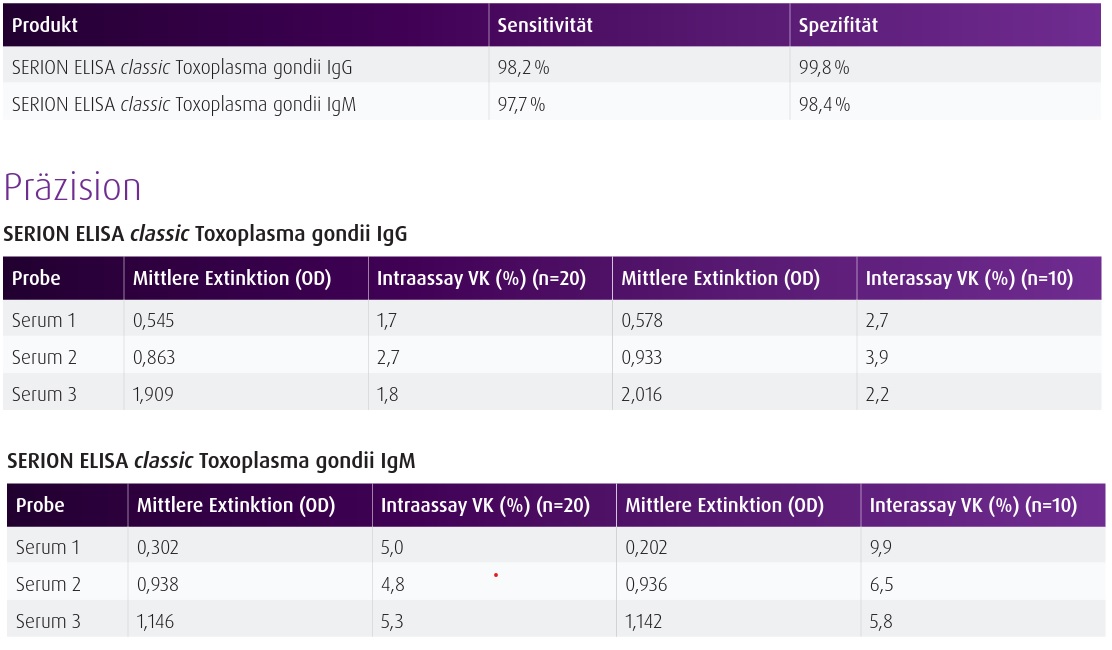
Background The SERION ELISA classic Toxoplasma gondii IgG and IgM tests are quantitative and qualitative immunoassays for the detection of human antibodies in serum or plasma directed against Toxoplasma gondii. The SERION ELISA classic Toxoplasma gondii IgG test allows for the determination of immune status as well as for the detection of intrathecally synthesized antibodies for CSF diagnostics and, by using the corresponding avidity reagent, of IgG antibody avidity determination in order to differentiate acute from past infections. The SERION ELISA classic Toxoplasma gondii IgM test serves as an initial assay for the detection of acute infections. The SERION ELISA classic Toxoplasma gondii IgM test is suitable for newborn screening with dried blood spots (DBS).
Sample Type Serum, plasma, whole blood
Assay Resolution Quantitative
Components
| Break apart microtiter test strips each with antigen coated single wells | 8 x 12 (96 Total) |
| Standard serum (ready-to-use) | 2 x 2 mL |
| Negative control serum (ready-to-use) | 2 mL |
| Anti-human-IgG-conjugate (ready-to-use) | 13 mL |
| Washing solution concentrate (sufficient for 1000ml) | 33.3 mL |
| Dilution buffer | 2 x 50 mL |
| Stopping solution | 15 mL |
| Substrate (ready-to-use) | 13 mL |
| Quality control certificate with standard curve and evaluation table | 1 |
Specificity Information
Target ID Human Toxoplasma gondii IgG
Research Areas Infectious Disease
Additional Information
Additional Information Additional Serion Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet