Mikrogen Diagnostik recomWell SARS-CoV-2 IgG ELISA Kit (7304)
$731.00
SKU: 7304
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomWell SARS-CoV-2 IgG ELISA Kit (7304)
Description Enzyme immunoassay with antigens produced by recombinant techniques for the detection of IgG and IgA antibodies against the coronavirus SARS-CoV-2 in human serum or plasma.
Product is intended for research use.
Product is intended for research use.
Target Human SARS-CoV-2 IgG
Species Reactivity SARS-CoV-2
Assay Type Indirect ELISA
Applications ELISA
Associated Products recomWell SARS-CoV-2 IgA ELISA Kit (7305)
recomLine SARS-CoV-2 IgG Lateral Strip Test Kit (7374)
SARS-CoV-2 IgA Agile ELISA Kit (ESR400A)
SARS-CoV-2 IgG Agile ELISA Kit (ESR400G)
SARS-CoV-2 IgM Agile ELISA Kit (ESR400M)
SARS-CoV-2 Spike Ectodomain (S1-S2) Antigen (BA400R03)
SARS-CoV-2 Nucleoprotein Antigen (BA400R04)
recomLine SARS-CoV-2 IgG Lateral Strip Test Kit (7374)
SARS-CoV-2 IgA Agile ELISA Kit (ESR400A)
SARS-CoV-2 IgG Agile ELISA Kit (ESR400G)
SARS-CoV-2 IgM Agile ELISA Kit (ESR400M)
SARS-CoV-2 Spike Ectodomain (S1-S2) Antigen (BA400R03)
SARS-CoV-2 Nucleoprotein Antigen (BA400R04)
Properties
Background In December 2019 began in the city of Wuhan, capital of Hubei in China, a pandemic spread of the disease caused by a new variant of the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV). The newly discovered variant is called SARS-CoV-2 and is closely related to SARS-CoV(-1). SARS coronaviruses spread primarily via droplets in exhaled air to transmit from person to person.Symptoms range from fever, cough and dyspnoea to pneumonia and acute respiratory distress syndrome and ultimately death in persons with comorbidities. Since the end of 2020, several vaccines against SARS-CoV-2 have been approved, most of which target the spike protein of the virus that mediates contact with the host cell.According to the German Robert Koch Institute, infected persons usually develop detectable antibodies in the second week after the onset of symptoms. A seroconversion or a significant increase in titer for IgG antibodies in the same test system can indicate an acute infection, especially in combination with corresponding symptoms. Thus, serological detection of antibodies serves as an ideal addition to molecular detection, which is recommended for acute diagnostics. Furthermore, the detection of IgG antibodies is a clear indication of pathogen contact and can detect a past infection and can be used for epidemiological studies.Product advantages for your benefitVery high sensitivity and specificity due to the use of highly purified recombinant nucleocapsid antigenEasy test procedure in the automatable ELISA screening format; quantitative resultsIdentical processing as well as uniform and interchangeable reagents for all MIKROGEN recomWell ELISABreak-aparts: single sample examination possibleCE label: The recomWell SARS-CoV-2 IgG, IgA tests meet the high standards of the European directive 98/79/EC on in vitro diagnostic medical devices
Sample Type Serum, plasma, whole blood
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| Dilution Buffer | 125 mL |
| TMB Substrate | 12 mL |
| Stop Slution | 12 mL |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Microplate Sealing Tape | 2 Each |
| Microtiter Plate | 12 x 8 Wells |
| Positive Control | 450 uL |
| Cut-Off Control | 450 uL |
| Negative Control | 450 uL |
| Anti-Human IgG Conjugate | 500 uL |
Specificity Information
Target ID Human SARS-CoV-2 IgG
Research Areas Infectious Disease
Application Images






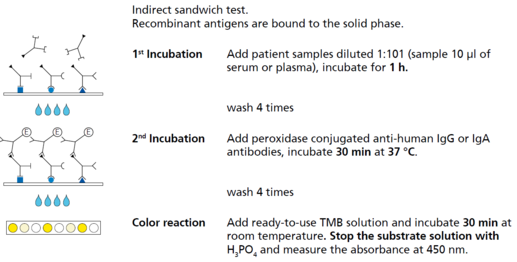
Description Test principle and procedure.

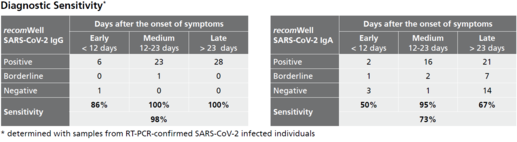
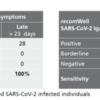
Description Sensitivity

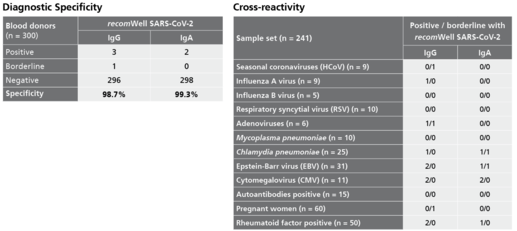
Description Specificity
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet