Mikrogen Diagnostik recomLine HTLV-1 & HTLV-2 IgG Lateral Strip Test Kit (5272)
$700.00
| Host | Quantity | Applications | Species Reactivity | Data Sheet | |
|---|---|---|---|---|---|
| 20 Tests | Lateral Strip Assay | T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) |  |
SKU: 5272
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomLine HTLV-1 & HTLV-2 IgG Lateral Strip Test Kit (5272)
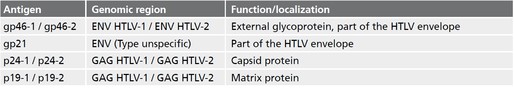
Description Strip-Immunoassay with antigens produced by recombinant techniques for the detection of IgG antibodies against human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2). Markers include:
p19-1
p19-2
p24-1
p24-2
gp46-1
gp46-2
gp21
p19-1
p19-2
p24-1
p24-2
gp46-1
gp46-2
gp21
Target Human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) IgG
Species Reactivity T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2)
Assay Type Indirect Sandwich ELISA
Applications Lateral Strip Assay
Properties
Background The human T-lymphotropic virus (HTLV) is a retrovirus, which can cause rare but severe diseases like adult T-cell leukemia (ATL) or HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP) in humans. Several HTLV types are known of which HTLV-1 and HTLV-2 are the most prevalent ones. It is possible to discriminate between HTLV-1 and HTLV-2 with molecular and serological diagnostic methods.
HTLV prevalence greatly varies by region and is highest in endemic areas like Japan, South America, the Caribbean and certain parts of Africa. In Europe a relevant prevalence can be found in countries with historical ties to these regions, but also for example in Romania. The total number of HTLV-infected persons can only be roughly estimated, the numbers are incomplete and range up to a total of more than 20 million cases worldwide.
The virus depends on a direct cell-cell contact to establish an infection. Due to this, blood plasma is not considered as an infective agent. Vertical mother to child transmission, especially during breastfeeding, is one of the most common routes of infection. Moreover, transmission through unprotected sexual contact, drug use and contaminated blood products or transplants is also relevant.
Since there is no curative therapy for HTLV, diagnostics is key to contain the virus. Only by the means of fast and reliable diagnostics the risk of vertical transmission through infected mothers can be reduced and safety of blood products and transplants can be guaranteed. Usually HTLV diagnostic is performed in two steps: A positive or ambiguous result of a serological screening test is followed by a confirmatory assay, such as a line immunoassay.
The recomLine HTLV-1 & HTLV-2 IgG is a qualitative test for the detection of IgG antibodies against HTLV-1 as well as HTLV-2 in human serum or plasma. The test is able to discriminate between virus type 1 and type 2 on one strip, therefore it is ideal for a simple and safe confirmation of screening results.
HTLV prevalence greatly varies by region and is highest in endemic areas like Japan, South America, the Caribbean and certain parts of Africa. In Europe a relevant prevalence can be found in countries with historical ties to these regions, but also for example in Romania. The total number of HTLV-infected persons can only be roughly estimated, the numbers are incomplete and range up to a total of more than 20 million cases worldwide.
The virus depends on a direct cell-cell contact to establish an infection. Due to this, blood plasma is not considered as an infective agent. Vertical mother to child transmission, especially during breastfeeding, is one of the most common routes of infection. Moreover, transmission through unprotected sexual contact, drug use and contaminated blood products or transplants is also relevant.
Since there is no curative therapy for HTLV, diagnostics is key to contain the virus. Only by the means of fast and reliable diagnostics the risk of vertical transmission through infected mothers can be reduced and safety of blood products and transplants can be guaranteed. Usually HTLV diagnostic is performed in two steps: A positive or ambiguous result of a serological screening test is followed by a confirmatory assay, such as a line immunoassay.
The recomLine HTLV-1 & HTLV-2 IgG is a qualitative test for the detection of IgG antibodies against HTLV-1 as well as HTLV-2 in human serum or plasma. The test is able to discriminate between virus type 1 and type 2 on one strip, therefore it is ideal for a simple and safe confirmation of screening results.
Sample Type Serum, plasma, whole blood
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| TMB Substrate | 40 mL |
| Milk Powder | 5 g |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Test Strips | 2 Vials x 10 Each |
| Anti-Human IgG Conjugate | 500 uL |
| Positive Control | 140 uL |
| Negative Control | 140 uL |
Specificity Information
Target Name HTLV-1 and HTLV-2 markers: p19-1, p19-2, p24-1, p24-2, gp46-1, gp46-2, gp21
Target ID Human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) IgG
Alternative Names p19-1, p19-2, p24-1, p24-2, gp46-1, gp46-2, gp21
Research Areas Infectious Disease
Application Images





Description Test Strip Layout

Description Test Principle

Description Evaluation

Description Evaluation
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet