Mikrogen Diagnostik recomLine Epstein-Barr Virus IgM Lateral Strip Test Kit (4573)
$660.00
| Host | Quantity | Applications | Species Reactivity | Data Sheet | |
|---|---|---|---|---|---|
| 20 Tests | Lateral Strip Assay | Epstein–Barr virus |  |
SKU: 4573
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomLine Epstein-Barr Virus IgM Lateral Strip Test Kit (4573)
Description Strip-Immunoassay with antigens produced by recombinant techniques for the detection of IgG, IgA and IgM antibodies against the Epstein-Barr virus (Epstein-Barr Virus)
Product is intended for research use.
Product is intended for research use.
Target Human Epstein–Barr virus IgM
Species Reactivity Epstein–Barr virus
Assay Type Indirect Sandwich ELISA
Applications Lateral Strip Assay
Associated Products recomLine Epstein-Barr Virus IgG Lateral Strip Test Kit (4572)
Epstein-Barr Virus VCA IgG Control (C1361G)
Epstein-Barr Virus VCA IgM Control (C1361M)
Epstein-Barr Virus EBNA-1 IgG Control Serum (C1362G)
Epstein-Barr Virus EA IgG Control (C1363G)
Epstein-Barr Virus/VCA IgG ELISA Kit (ESR1361G)
Epstein-Barr Virus/VCA IgM ELISA Kit (ESR1361M)
Epstein-Barr Virus/EBNA1 IgG ELISA Kit (ESR1362G)
Epstein-Barr Virus EA IgG ELISA Kit (ESR1363G)
Epstein Barr Virus (EBV) Capsid Antigen P18 Antigen (BA1361VSR22)
Epstein Barr Virus (EBV) Capsid Antigen P23 Antigen (BA1361VSR21)
Epstein Barr Virus (EBV) Nuclear Antigen EBNA1, P72 Antigen (BA1362VS)
Epstein Barr Virus (EBV) Early Antigen P54 Antigen (BA1363P54VS)
Epstein Barr Virus (EBV) Early Antigen P138 Antigen (BA1363VSR6)
Epstein-Barr Virus VCA IgG Control (C1361G)
Epstein-Barr Virus VCA IgM Control (C1361M)
Epstein-Barr Virus EBNA-1 IgG Control Serum (C1362G)
Epstein-Barr Virus EA IgG Control (C1363G)
Epstein-Barr Virus/VCA IgG ELISA Kit (ESR1361G)
Epstein-Barr Virus/VCA IgM ELISA Kit (ESR1361M)
Epstein-Barr Virus/EBNA1 IgG ELISA Kit (ESR1362G)
Epstein-Barr Virus EA IgG ELISA Kit (ESR1363G)
Epstein Barr Virus (EBV) Capsid Antigen P18 Antigen (BA1361VSR22)
Epstein Barr Virus (EBV) Capsid Antigen P23 Antigen (BA1361VSR21)
Epstein Barr Virus (EBV) Nuclear Antigen EBNA1, P72 Antigen (BA1362VS)
Epstein Barr Virus (EBV) Early Antigen P54 Antigen (BA1363P54VS)
Epstein Barr Virus (EBV) Early Antigen P138 Antigen (BA1363VSR6)
Properties
Background The Epstein-Barr virus, an ubiquitously occurring herpes virus, can cause the symptoms of infectious mononucleosis (Pfeiffer's disease) on primary infection. Moreover, as a result of the lifelong persistence of this pathogen, reactivations can occur, especially in immuno-incompetent persons.Due to the diversity of symptoms caused by primary infection or reactivation and their correspondence with the symptoms of other diseases, one of the main tasks in routine diagnosis is the serological detection of a primary infection, past infection or possible reactivation. For this purpose, a series of individual determinations (EIA and IFT) are generally carried out for the particular class of antigen and type of antibody.The recomLine Epstein-Barr Virus, with the antigens sprayed onto the nitrocellulose, is designed as screening immunoassay. The line-assay technique allows the detection and identification of IgG and IgM antibodies directed against the different Epstein-Barr Virus antigen classes in a single approach. The application of highly specific and characteristic Epstein-Barr Virus proteins is made possible by the use of antigens produced by genetic engineering."The combination of p18 and EBNA-1 (in IgG detection) represents a so far unrivalled degree of certainty in the exclusion of primary infections ..."Prof. Dr. G. Bauer, Freiburg '99
Sample Type Serum, plasma, whole blood
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| TMB Substrate | 40 mL |
| Milk Powder | 5 g |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Test Strips | 2 Vials x 10 Each |
| Anti-Human IgM Conjugate | 500 uL |
Specificity Information
Target ID Human Epstein–Barr virus IgM
Research Areas Infectious Disease
Application Images






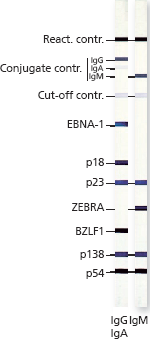
Description Strip layout.

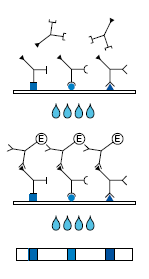
Description Test Procedure:
1. 1st Incubation. A test strip loaded with EBV antigens is incubated with diluted serum or plasma in a dish for 1 hour.
2. Wash 3 times
3. 2nd Incubation
4. Peroxidase conjugated anti-human antibodies (IgG, IgA or IgM specific) are added. Incubate for 45 minutes.
5. Wash 3 times
6. Color reaction
7. 8 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies.
1. 1st Incubation. A test strip loaded with EBV antigens is incubated with diluted serum or plasma in a dish for 1 hour.
2. Wash 3 times
3. 2nd Incubation
4. Peroxidase conjugated anti-human antibodies (IgG, IgA or IgM specific) are added. Incubate for 45 minutes.
5. Wash 3 times
6. Color reaction
7. 8 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies.

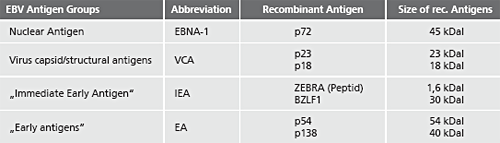
Description Recombinant EBV Antigens used in the Test

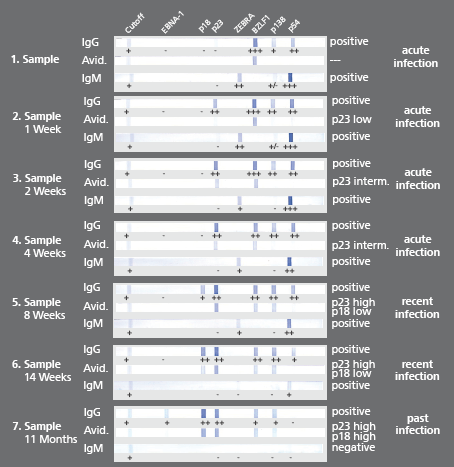
Description Example data:
-BZLF1 IgG and ZEBRA IgM appear as early markers beside the EA antigens (IgG and IgM)
-p18 IgG appears as late marker in the 8th week (sample 5)
-Avidity of p23 IgG rises during approx. 7 weeks (sample 2-5)
-EBNA-1 IgG appears as late marker after 11 months (sample 7)
-BZLF1 IgG and ZEBRA IgM appear as early markers beside the EA antigens (IgG and IgM)
-p18 IgG appears as late marker in the 8th week (sample 5)
-Avidity of p23 IgG rises during approx. 7 weeks (sample 2-5)
-EBNA-1 IgG appears as late marker after 11 months (sample 7)
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet