Mikrogen Diagnostik recomLine Cytomegalovirus IgM Lateral Strip Test Kit (5573)
$660.00
| Host | Quantity | Applications | Species Reactivity | Data Sheet | |
|---|---|---|---|---|---|
| 20 Tests | Lateral Strip Assay | Cytomegalovirus |  |
SKU: 5573
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomLine Cytomegalovirus IgM Lateral Strip Test Kit (5573)
Description Line immunoassay using recombinant antigens to detect IgG and IgM antibodies directed against Cytomegalovirus (Cytomegalovirus) and to determine Cytomegalovirus-IgG avidity Cytomegalovirus (Cytomegalovirus)
Product is intended for research use.
Product is intended for research use.
Target Human Cytomegalovirus IgM
Species Reactivity Cytomegalovirus
Assay Type Indirect Sandwich ELISA
Applications Lateral Strip Assay
Associated Products Cytomegalovirus Antigen (BA109VS)
recomLine Cytomegalovirus IgG (Avidity) Lateral Strip Test Kit (5572)
Cytomegalovirus IgG Control (C109G)
Cytomegalovirus IgM Control (C109M)
Cytomegalovirus IgG ELISA Kit (ESR109G)
Cytomegalovirus IgM ELISA Kit (ESR109M)
Cytomegalovirus Avidity Control IgG (BR109AVID)
recomLine Cytomegalovirus IgG (Avidity) Lateral Strip Test Kit (5572)
Cytomegalovirus IgG Control (C109G)
Cytomegalovirus IgM Control (C109M)
Cytomegalovirus IgG ELISA Kit (ESR109G)
Cytomegalovirus IgM ELISA Kit (ESR109M)
Cytomegalovirus Avidity Control IgG (BR109AVID)
Properties
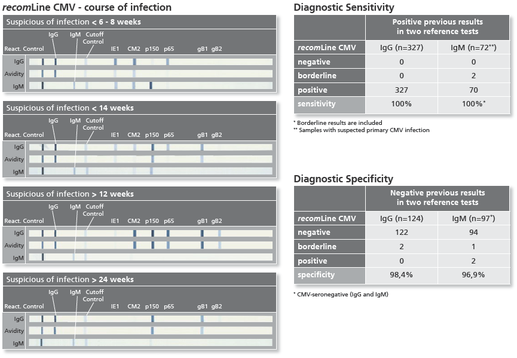
Background Infections with human cytomegalovirus (Cytomegalovirus, HHV5) normally cause mild symptoms, many patients even remain asymptomatically. Immunocompromised patients and pregnant women however are at high risk for suffering damage from a Cytomegalovirus infection. Under immunosuppressive conditions Cytomegalovirus infection or reactivation can cause a severe clinical course of disease. Pregnant women acquiring a primary infection in approximately 40 percent of the cases transmit the virus to the fetus. About 10 percent of the infected newborns show severe damage at birth or are going to develop long-term effects. Cytomegalovirus infection in early pregnancy may lead to abortion or severe clinical symptoms of the affected children, whereas infection in late pregnancy mostly results in mild cases of illness, or even in asymptomatic newborns.The recomLine Cytomegalovirus is a line immunoassay using all diagnostic relevant Cytomegalovirus antigens in a recombinant format optimized to highest performance in sensitivity and specificity at the same time. Purpose of the assay is the confirmation of positive and unclear screening results (confirmatory assay). Especially the low predictive value of positive IgM results due to persistent or recurrent infections has to be clarified. The test system either is able to determine IgG and IgM antibodies and the avidity of IgG antibodies. Using phase-specific antigens and separate avidity antigens (patented method from MIKROGEN on line immunoassays) the test system can detect Cytomegalovirus specific antibodies in all phases of the infection. Despite viral load the recomLine Cytomegalovirus can be used in pregnancy diagnostic or pre-surgical diagnostic of transplant settings to precisely state if the patient can be judged acute infected or shows typical antigen patterns of a long past infection. While supporting a clear answer to the question what impact the detected Cytomegalovirus antibodies for this particular patient have, this assay can help to optimize therapy, improve disease burden and give patient and doctor relief on reduced acuteness.
Sample Type Serum, plasma, whole blood
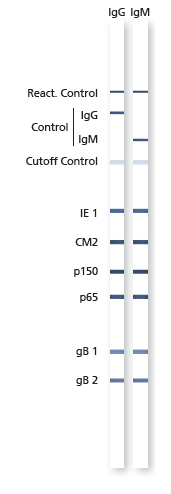
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| TMB Substrate | 40 mL |
| Milk Powder | 5 g |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Test Strips | 2 Vials x 10 Each |
| Anti-Human IgM Conjugate | 500 uL |
Specificity Information
Target ID Human Cytomegalovirus IgM
Research Areas Infectious Disease
Application Images







Description Strip layout.

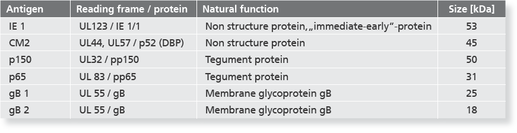
Description Recombinant CMV Antigens.

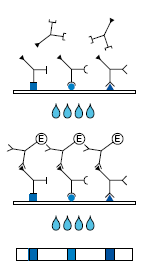
Description Test Principle and Procedure.
1. 1st Incubation. A test strip loaded with CMV antigens is incubated with diluted serum or plasma in a dish for 1 hour.
2. Wash 3 times
3. 2nd Incubation
4. Peroxidase conjugated anti-human antibodies (IgG or IgM specific) are added. Incubate for 45 minutes.
5. wash 3 times
6. Color reaction. 8 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies.
1. 1st Incubation. A test strip loaded with CMV antigens is incubated with diluted serum or plasma in a dish for 1 hour.
2. Wash 3 times
3. 2nd Incubation
4. Peroxidase conjugated anti-human antibodies (IgG or IgM specific) are added. Incubate for 45 minutes.
5. wash 3 times
6. Color reaction. 8 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies.

Description Test evaluation, sensitivity and specificity.
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet