BriClone Hybridoma Cloning Medium (BRI10000)
$368.00
Note: This product has a $35.00 Dry Ice Fee.
Overview
Product Name BriClone Hybridoma Cloning Medium (BRI10000)
Description BriClone is an additive for the cloning medium used in the post-fusion stages of hybridoma production and for improving the efficiency of hybridoma cells cloning.
Applications Cell Culture
Associated Products BriClone Serum Free Hybirdoma Cloning Medium
Properties
Form Liquid
Concentration 20X Solution
Formulation Dulbeccos Modified Eagle medium (DMEM): Non Animal Source
Foetal Bovine Serum: origin United States of America, certified free of viruses
Foetal Bovine Serum: origin United States of America, certified free of viruses
Background BriClone completely replaces the need for feeder cells. The use of feeder cells to support the outgrowth of hybridomas has many disadvantages, like batch-to-batch variation of the feeder cells and competition between feeder cells and freshly-fused hybridomas for nutrients in the culture medium. BriClone has been specially developed to overcome these common problems in the post-fusion period.
Application Images






Description Hybridoma cloning efficiency using BriClone or mouse macrophages (feeder). The hybridomas were cloned by limiting dilution at 1 cell/well.

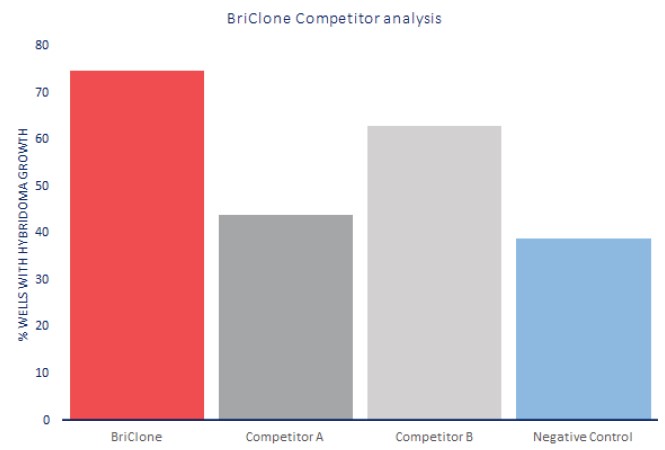
Description Many commercial additives are available for improving the improving the efficiency of cell cloning at the post-fusion stages of hybridoma production. In the following experiment, BriClone was compared to two competitors as a supplement for post-fusion hybridoma production by assessing fusion efficiency. Fusion efficiency is expressed as a percentage of wells in 48-well plates with positive hybridoma clones following fusion, relative to BriClone positive control (thawed overnight). All supplements used at their recommended concentrations. BriClone increased the number of positive wells identified with hybridoma clones, when compared to two separate competitors. Negative control indicates no supplement added.

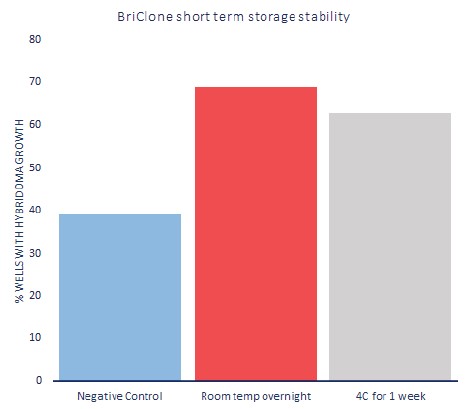
Description To demonstrate the stability of BriClone, various simulated conditions were assessed mimicking different temperature exposures. Post-fusion hybridoma production was assessed using BriClone under different conditions (overnight at room temperature) as a positive control, as well as one week stored at 4°C. Fusion efficiency is expressed as a percentage of wells in 48-well plates with positive hybridoma clones following fusion. No notable difference was observed in hybridoma yield between use of BriClone thawed overnight at room temperature or after being stored for one week at 4˚C. Stability of BriClone is therefore not significantly affected following exposure to extended ambient temperatures as may occasionally be observed in transport of the product on ice packs. Negative control was performed without any supplement.
Additional Information
Additional Information Sterility:
Each batch of BriClone undergoes a filtration using a Supor® membrane(hydrophilic polyesthersulphone (PES) of 0.2μm)
Each batch is tested for absence of detectable microbial contamination, as follows:
-Aerobic and anaerobic bacteria (Thioglycollate Broth at 37˚C for 15 days )
-Fungi (Trypton Soy Broth at 25ºC for 15 days)
-Mycoplasma (Hoechst direct staining method)
Quality Control:
Each batch of BriClone is tested for its ability to support the outgrowth of hybridomas from freshly fused cells. Test cells used are SP2/O-Ag14 and immune splenocytes from BALB/c mice. Hybridomas are cloned by limiting dilution under conditions designed to seed one cell/well in 800μl on 48-well plates over a 12 days incubation period.
-This product is for in vitro research purposes only. Not for human or veterinary use.
-Always use BriClone under aseptic conditions
-This product has been produced using cells that have not been screened for Hepatitis B, Human immunodeficiency viruses or other agents.
-Handle as a potentially biohazardous material under at least Biosafety Level 1 containment.
Each batch of BriClone undergoes a filtration using a Supor® membrane(hydrophilic polyesthersulphone (PES) of 0.2μm)
Each batch is tested for absence of detectable microbial contamination, as follows:
-Aerobic and anaerobic bacteria (Thioglycollate Broth at 37˚C for 15 days )
-Fungi (Trypton Soy Broth at 25ºC for 15 days)
-Mycoplasma (Hoechst direct staining method)
Quality Control:
Each batch of BriClone is tested for its ability to support the outgrowth of hybridomas from freshly fused cells. Test cells used are SP2/O-Ag14 and immune splenocytes from BALB/c mice. Hybridomas are cloned by limiting dilution under conditions designed to seed one cell/well in 800μl on 48-well plates over a 12 days incubation period.
-This product is for in vitro research purposes only. Not for human or veterinary use.
-Always use BriClone under aseptic conditions
-This product has been produced using cells that have not been screened for Hepatitis B, Human immunodeficiency viruses or other agents.
-Handle as a potentially biohazardous material under at least Biosafety Level 1 containment.
Handling
Storage Store at -20°C. Avoid repeated freeze/thaw cycles. Stable for short periods at +4°C
Dilution Instructions Thaw and add to the hybridoma cloning medium as a 5% v/v supplement.
Application Instructions Thaw and add to the hybridoma cloning medium as a 5% v/v supplement.
BriClone SF can be used for:
• Hybridoma growth post-fusion (refer to your own protocol)
• Hybridoma Cloning (refer to your own protocol)
• Hybridoma growth post-fusion (refer to your own protocol)
• Hybridoma Cloning (refer to your own protocol)
References & Data Sheet
References PMC7873262,PMC7239657,PMC6803335,PMC6816435,PMC6719973,PMC5017023,PMC2812581,PMC2896538,PMC4617144,PMC3944398,PMC9030397,PMC8470961,PMC8104110,PMC7279471,PMC7096428,PMC5992998,PMC5986985,PMC5857169,PMC4921032,PMC4591809,PMC4135088,PMC1858089,PMC1602225
PMID Purification of anti-glycoconjugate monoclonal antibodies using newly developed porous zirconia particles
Tetsuya Okuda, Katsuya Kato, Masahiro Kitamura, Shinjiro Kasahara
Sci Rep. 2021; 11: 3233. Published online 2021 Feb 9. doi: 10.1038/s41598-021-82457-0
Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion Julia Damiano-Guercio, Laëtitia Kurzawa, Jan Mueller, Georgi Dimchev, Matthias Schaks, Maria Nemethova, Thomas Pokrant, Stefan Brühmann, Joern Linkner, Laurent Blanchoin, Michael Sixt, Klemens Rottner, Jan Faix eLife. 2020; 9: e55351. Published online 2020 May 11. doi: 10.7554/eLife.55351
Elucidation of the Interleukin 12 Production Mechanism during Intracellular Bacterial Infection in Amberjack, Seriola dumerili Megumi Matsumoto, Taisei Kubota, Sinsuke Fujita, Kazuhiro Shiozaki, Shosei Kishida, Atsushi Yamamoto Infect Immun. 2019 Nov; 87(11): e00459-19. Prepublished online 2019 Sep 9. Published online 2019 Oct 18. doi: 10.1128/IAI.00459-19
Characterization of novel anti-IL-26 neutralizing monoclonal antibodies for the treatment of inflammatory diseases including psoriasis Ryo Hatano, Takumi Itoh, Haruna Otsuka, Sayo Okamoto, Eriko Komiya, Satoshi Iwata, Thomas M. Aune, Nam H. Dang, Kyoko Kuwahara-Arai, Kei Ohnuma, Chikao Morimoto MAbs. 2019 Nov-Dec; 11(8): 1428–1442. Published online 2019 Aug 18. doi: 10.1080/19420862.2019.1654305
Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II Yoshiro Hanyu, Yuto Komeiji, Mieko Kato Molecules. 2019 Aug; 24(16): 2949. Published online 2019 Aug 14. doi: 10.3390/molecules24162949
Probing conformational and functional states of human hepatocyte growth factor by a panel of monoclonal antibodies Masataka Umitsu, Katsuya Sakai, Satoshi Ogasawara, Mika K. Kaneko, Ryoko Asaki, Keiko Tamura-Kawakami, Yukinari Kato, Kunio Matsumoto, Junichi Takagi Sci Rep. 2016; 6: 33149. Published online 2016 Sep 9. doi: 10.1038/srep33149
GMab-1, a high-affinity anti-3'-isoLM1/3',6'-isoLD1 IgG monoclonal antibody, raised in lacto-series ganglioside-defective knockout mice Yukinari Kato, Chien-Tsun Kuan, Jinli Chang, Mika Kato Kaneko, Joanne Ayriss, Hailan Piao, Vidyalakshmi Chandramohan, Charles Pegram, Roger E. McLendon, Pam Fredman, Jan-Eric Månsson, Darell D. Bigner Biochem Biophys Res Commun. Author manuscript; available in PMC 2011 Jan 1.Published in final edited form as: Biochem Biophys Res Commun. 2010 Jan 1; 391(1): 750–755. Published online 2009 Nov 26. doi: 10.1016/j.bbrc.2009.11.132
Quantitative detection of 4-hydroxyequilenin–DNA adducts in mammalian cells using an immunoassay with a novel monoclonal antibody Yumiko Okahashi, Takaaki Iwamoto, Naomi Suzuki, Shinya Shibutani, Shigeki Sugiura, Shinji Itoh, Tomohisa Nishiwaki, Satoshi Ueno, Toshio Mori Nucleic Acids Res. 2010 Jul; 38(12): e133. Published online 2010 Apr 20. doi: 10.1093/nar/gkq233
Direct detection of ABCA1-dependent HDL formation based on lipidation-induced hydrophobicity change in apoA-I Risa Omura, Kohjiro Nagao, Norihiro Kobayashi, Kazumitsu Ueda, Hiroyuki Saito J Lipid Res. 2014 Nov; 55(11): 2423–2431. doi: 10.1194/jlr.D049445
Establishment of monoclonal anti-human CD26 antibodies suitable for immunostaining of formalin-fixed tissue Ryo Hatano, Taketo Yamada, Shuji Matsuoka, Satoshi Iwata, Hiroto Yamazaki, Eriko Komiya, Toshihiro Okamoto, Nam H Dang, Kei Ohnuma, Chikao Morimoto Diagn Pathol. 2014; 9: 30. Published online 2014 Feb 6. doi: 10.1186/1746-1596-9-30
Development and Characterization of Anti-Naja ashei Three-Finger Toxins (3FTxs)-Specific Monoclonal Antibodies and Evaluation of Their In Vitro Inhibition Activity Ernest Z. Manson, Mutinda C. Kyama, Josephine Kimani, Aleksandra Bocian, Konrad K. Hus, Vladimír Petrilla, Jaroslav Legáth, James H. Kimotho Toxins (Basel) 2022 Apr; 14(4): 285. Published online 2022 Apr 16. doi: 10.3390/toxins14040285
Biallelic SYNE2 Missense Mutations Leading to Nesprin-2 Giant Hypo-Expression Are Associated with Intellectual Disability and Autism Natalie Young, Maria Asif, Matthew Jackson, Daniel Martín Fernández-Mayoralas, Mar Jimenez de la Peña, Beatriz Calleja-Pérez, Sara Álvarez, Eve Hunter-Featherstone, Angelika A. Noegel, Wolfgang Höhne, Peter Nürnberg, Boguslaw Obara, Muhammad Sajid Hussain, Iakowos Karakesisoglou, Alberto Fernández-Jaén Genes (Basel) 2021 Sep; 12(9): 1294. Published online 2021 Aug 24. doi: 10.3390/genes12091294
Antibody Screening System Using a Herpes Simplex Virus (HSV)-Based Probe To Identify a Novel Target for Receptor-Retargeted Oncolytic HSVs Hitomi Ikeda, Hiroaki Uchida, Yu Okubo, Tomoko Shibata, Yasuhiko Sasaki, Takuma Suzuki, Mika Hamada-Uematsu, Ryota Hamasaki, Kosaku Okuda, Miki Yamaguchi, Masaki Kojima, Masato Tanaka, Hirofumi Hamada, Hideaki Tahara J Virol. 2021 May; 95(9): e01766-20. Prepublished online 2021 Feb 24. Published online 2021 Apr 12. doi: 10.1128/JVI.01766-20
Isolation and Characterization of Antibodies Induced by Immunization with TNF-α Inducible Globotetraosylceramide Tetsuya Okuda Int J Mol Sci. 2020 May; 21(10): 3632. Published online 2020 May 21. doi: 10.3390/ijms21103632
CDK1 dependent phosphorylation of hTERT contributes to cancer progression Mami Yasukawa, Yoshinari Ando, Taro Yamashita, Yoko Matsuda, Shisako Shoji, Masaki Suimye Morioka, Hideya Kawaji, Kumiko Shiozawa, Mitsuhiro Machitani, Takaya Abe, Shinji Yamada, Mika K. Kaneko, Yukinari Kato, Yasuhide Furuta, Tadashi Kondo, Mikako Shirouzu, Yoshihide Hayashizaki, Shuichi Kaneko, Kenkichi Masutomi Nat Commun. 2020; 11: 1557. Published online 2020 Mar 25. doi: 10.1038/s41467-020-15289-7
Data on immunoglobulin G antibodies induced by immunization of mice with globoside carrying very long-chain fatty acids Tetsuya Okuda Data Brief. 2018 Aug; 19: 256–260. Published online 2018 May 10. doi: 10.1016/j.dib.2018.05.014
Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C44Mab-5 Shinji Yamada, Shunsuke Itai, Takuro Nakamura, Miyuki Yanaka, Mika K. Kaneko, Yukinari Kato Biochem Biophys Rep. 2018 Jul; 14: 64–68. Published online 2018 Apr 12. doi: 10.1016/j.bbrep.2018.03.007
Detection of high PD-L1 expression in oral cancers by a novel monoclonal antibody L1Mab-4 Shinji Yamada, Shunsuke Itai, Mika K. Kaneko, Yukinari Kato Biochem Biophys Rep. 2018 Mar; 13: 123–128. Published online 2018 Feb 6. doi: 10.1016/j.bbrep.2018.01.009
Establishment of anti-mesothelioma monoclonal antibodies Natsuko Mizutani, Masaaki Abe, Shuji Matsuoka, Kazunori Kajino, Midori Wakiya, Naomi Ohtsuji, Ryo Hatano, Chikao Morimoto, Okio Hino BMC Res Notes. 2016; 9: 324. Published online 2016 Jun 24. doi: 10.1186/s13104-016-2128-x
Cellular Interaction and Cytotoxicity of the Iowa Mutation of Apolipoprotein A-I (ApoA-IIowa) Amyloid Mediated by Sulfate Moieties of Heparan Sulfate Kaori Kuwabara, Kazuchika Nishitsuji, Kenji Uchimura, Shang-Cheng Hung, Makoto Mizuguchi, Hiroyuki Nakajima, Shiho Mikawa, Norihiro Kobayashi, Hiroyuki Saito, Naomi Sakashita J Biol Chem. 2015 Oct 2; 290(40): 24210–24221. Published online 2015 Aug 19. doi: 10.1074/jbc.M115.652545
Newly isolated mAbs broaden the neutralizing epitope in murine norovirus Abimbola O. Kolawole, Chunsheng Xia, Ming Li, Monica Gamez, Chenchen Yu, Christine M. Rippinger, Ryan E. Yucha, Thomas J. Smith, Christiane E. Wobus J Gen Virol. 2014 Sep; 95(Pt 9): 1958–1968. doi: 10.1099/vir.0.066753-0
Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Y. Shinmura, S. Aiba-Masago, I. Kosugi, R. Y. Li, S. Baba, Y. Tsutsui Am J Pathol. 1997 Nov; 151(5): 1331–1340.
Increased Expression of a Myc Target Gene Mina53 in Human Colon Cancer Kwesi Teye, Makoto Tsuneoka, Nobuyuki Arima, Yoshiro Koda, Yasuhiro Nakamura, Yoichi Ueta, Kazuo Shirouzu, Hiroshi Kimura Am J Pathol. 2004 Jan; 164(1): 205–216. doi: 10.1016/S0002-9440(10)63111-2
Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion Julia Damiano-Guercio, Laëtitia Kurzawa, Jan Mueller, Georgi Dimchev, Matthias Schaks, Maria Nemethova, Thomas Pokrant, Stefan Brühmann, Joern Linkner, Laurent Blanchoin, Michael Sixt, Klemens Rottner, Jan Faix eLife. 2020; 9: e55351. Published online 2020 May 11. doi: 10.7554/eLife.55351
Elucidation of the Interleukin 12 Production Mechanism during Intracellular Bacterial Infection in Amberjack, Seriola dumerili Megumi Matsumoto, Taisei Kubota, Sinsuke Fujita, Kazuhiro Shiozaki, Shosei Kishida, Atsushi Yamamoto Infect Immun. 2019 Nov; 87(11): e00459-19. Prepublished online 2019 Sep 9. Published online 2019 Oct 18. doi: 10.1128/IAI.00459-19
Characterization of novel anti-IL-26 neutralizing monoclonal antibodies for the treatment of inflammatory diseases including psoriasis Ryo Hatano, Takumi Itoh, Haruna Otsuka, Sayo Okamoto, Eriko Komiya, Satoshi Iwata, Thomas M. Aune, Nam H. Dang, Kyoko Kuwahara-Arai, Kei Ohnuma, Chikao Morimoto MAbs. 2019 Nov-Dec; 11(8): 1428–1442. Published online 2019 Aug 18. doi: 10.1080/19420862.2019.1654305
Potentiating Antigen-Specific Antibody Production with Peptides Obtained from In Silico Screening for High-Affinity against MHC-II Yoshiro Hanyu, Yuto Komeiji, Mieko Kato Molecules. 2019 Aug; 24(16): 2949. Published online 2019 Aug 14. doi: 10.3390/molecules24162949
Probing conformational and functional states of human hepatocyte growth factor by a panel of monoclonal antibodies Masataka Umitsu, Katsuya Sakai, Satoshi Ogasawara, Mika K. Kaneko, Ryoko Asaki, Keiko Tamura-Kawakami, Yukinari Kato, Kunio Matsumoto, Junichi Takagi Sci Rep. 2016; 6: 33149. Published online 2016 Sep 9. doi: 10.1038/srep33149
GMab-1, a high-affinity anti-3'-isoLM1/3',6'-isoLD1 IgG monoclonal antibody, raised in lacto-series ganglioside-defective knockout mice Yukinari Kato, Chien-Tsun Kuan, Jinli Chang, Mika Kato Kaneko, Joanne Ayriss, Hailan Piao, Vidyalakshmi Chandramohan, Charles Pegram, Roger E. McLendon, Pam Fredman, Jan-Eric Månsson, Darell D. Bigner Biochem Biophys Res Commun. Author manuscript; available in PMC 2011 Jan 1.Published in final edited form as: Biochem Biophys Res Commun. 2010 Jan 1; 391(1): 750–755. Published online 2009 Nov 26. doi: 10.1016/j.bbrc.2009.11.132
Quantitative detection of 4-hydroxyequilenin–DNA adducts in mammalian cells using an immunoassay with a novel monoclonal antibody Yumiko Okahashi, Takaaki Iwamoto, Naomi Suzuki, Shinya Shibutani, Shigeki Sugiura, Shinji Itoh, Tomohisa Nishiwaki, Satoshi Ueno, Toshio Mori Nucleic Acids Res. 2010 Jul; 38(12): e133. Published online 2010 Apr 20. doi: 10.1093/nar/gkq233
Direct detection of ABCA1-dependent HDL formation based on lipidation-induced hydrophobicity change in apoA-I Risa Omura, Kohjiro Nagao, Norihiro Kobayashi, Kazumitsu Ueda, Hiroyuki Saito J Lipid Res. 2014 Nov; 55(11): 2423–2431. doi: 10.1194/jlr.D049445
Establishment of monoclonal anti-human CD26 antibodies suitable for immunostaining of formalin-fixed tissue Ryo Hatano, Taketo Yamada, Shuji Matsuoka, Satoshi Iwata, Hiroto Yamazaki, Eriko Komiya, Toshihiro Okamoto, Nam H Dang, Kei Ohnuma, Chikao Morimoto Diagn Pathol. 2014; 9: 30. Published online 2014 Feb 6. doi: 10.1186/1746-1596-9-30
Development and Characterization of Anti-Naja ashei Three-Finger Toxins (3FTxs)-Specific Monoclonal Antibodies and Evaluation of Their In Vitro Inhibition Activity Ernest Z. Manson, Mutinda C. Kyama, Josephine Kimani, Aleksandra Bocian, Konrad K. Hus, Vladimír Petrilla, Jaroslav Legáth, James H. Kimotho Toxins (Basel) 2022 Apr; 14(4): 285. Published online 2022 Apr 16. doi: 10.3390/toxins14040285
Biallelic SYNE2 Missense Mutations Leading to Nesprin-2 Giant Hypo-Expression Are Associated with Intellectual Disability and Autism Natalie Young, Maria Asif, Matthew Jackson, Daniel Martín Fernández-Mayoralas, Mar Jimenez de la Peña, Beatriz Calleja-Pérez, Sara Álvarez, Eve Hunter-Featherstone, Angelika A. Noegel, Wolfgang Höhne, Peter Nürnberg, Boguslaw Obara, Muhammad Sajid Hussain, Iakowos Karakesisoglou, Alberto Fernández-Jaén Genes (Basel) 2021 Sep; 12(9): 1294. Published online 2021 Aug 24. doi: 10.3390/genes12091294
Antibody Screening System Using a Herpes Simplex Virus (HSV)-Based Probe To Identify a Novel Target for Receptor-Retargeted Oncolytic HSVs Hitomi Ikeda, Hiroaki Uchida, Yu Okubo, Tomoko Shibata, Yasuhiko Sasaki, Takuma Suzuki, Mika Hamada-Uematsu, Ryota Hamasaki, Kosaku Okuda, Miki Yamaguchi, Masaki Kojima, Masato Tanaka, Hirofumi Hamada, Hideaki Tahara J Virol. 2021 May; 95(9): e01766-20. Prepublished online 2021 Feb 24. Published online 2021 Apr 12. doi: 10.1128/JVI.01766-20
Isolation and Characterization of Antibodies Induced by Immunization with TNF-α Inducible Globotetraosylceramide Tetsuya Okuda Int J Mol Sci. 2020 May; 21(10): 3632. Published online 2020 May 21. doi: 10.3390/ijms21103632
CDK1 dependent phosphorylation of hTERT contributes to cancer progression Mami Yasukawa, Yoshinari Ando, Taro Yamashita, Yoko Matsuda, Shisako Shoji, Masaki Suimye Morioka, Hideya Kawaji, Kumiko Shiozawa, Mitsuhiro Machitani, Takaya Abe, Shinji Yamada, Mika K. Kaneko, Yukinari Kato, Yasuhide Furuta, Tadashi Kondo, Mikako Shirouzu, Yoshihide Hayashizaki, Shuichi Kaneko, Kenkichi Masutomi Nat Commun. 2020; 11: 1557. Published online 2020 Mar 25. doi: 10.1038/s41467-020-15289-7
Data on immunoglobulin G antibodies induced by immunization of mice with globoside carrying very long-chain fatty acids Tetsuya Okuda Data Brief. 2018 Aug; 19: 256–260. Published online 2018 May 10. doi: 10.1016/j.dib.2018.05.014
Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C44Mab-5 Shinji Yamada, Shunsuke Itai, Takuro Nakamura, Miyuki Yanaka, Mika K. Kaneko, Yukinari Kato Biochem Biophys Rep. 2018 Jul; 14: 64–68. Published online 2018 Apr 12. doi: 10.1016/j.bbrep.2018.03.007
Detection of high PD-L1 expression in oral cancers by a novel monoclonal antibody L1Mab-4 Shinji Yamada, Shunsuke Itai, Mika K. Kaneko, Yukinari Kato Biochem Biophys Rep. 2018 Mar; 13: 123–128. Published online 2018 Feb 6. doi: 10.1016/j.bbrep.2018.01.009
Establishment of anti-mesothelioma monoclonal antibodies Natsuko Mizutani, Masaaki Abe, Shuji Matsuoka, Kazunori Kajino, Midori Wakiya, Naomi Ohtsuji, Ryo Hatano, Chikao Morimoto, Okio Hino BMC Res Notes. 2016; 9: 324. Published online 2016 Jun 24. doi: 10.1186/s13104-016-2128-x
Cellular Interaction and Cytotoxicity of the Iowa Mutation of Apolipoprotein A-I (ApoA-IIowa) Amyloid Mediated by Sulfate Moieties of Heparan Sulfate Kaori Kuwabara, Kazuchika Nishitsuji, Kenji Uchimura, Shang-Cheng Hung, Makoto Mizuguchi, Hiroyuki Nakajima, Shiho Mikawa, Norihiro Kobayashi, Hiroyuki Saito, Naomi Sakashita J Biol Chem. 2015 Oct 2; 290(40): 24210–24221. Published online 2015 Aug 19. doi: 10.1074/jbc.M115.652545
Newly isolated mAbs broaden the neutralizing epitope in murine norovirus Abimbola O. Kolawole, Chunsheng Xia, Ming Li, Monica Gamez, Chenchen Yu, Christine M. Rippinger, Ryan E. Yucha, Thomas J. Smith, Christiane E. Wobus J Gen Virol. 2014 Sep; 95(Pt 9): 1958–1968. doi: 10.1099/vir.0.066753-0
Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Y. Shinmura, S. Aiba-Masago, I. Kosugi, R. Y. Li, S. Baba, Y. Tsutsui Am J Pathol. 1997 Nov; 151(5): 1331–1340.
Increased Expression of a Myc Target Gene Mina53 in Human Colon Cancer Kwesi Teye, Makoto Tsuneoka, Nobuyuki Arima, Yoshiro Koda, Yasuhiro Nakamura, Yoichi Ueta, Kazuo Shirouzu, Hiroshi Kimura Am J Pathol. 2004 Jan; 164(1): 205–216. doi: 10.1016/S0002-9440(10)63111-2
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet