News from QED Bioscience
How to Choose Quality Antibodies for Successful Western Blotting
Successful western blotting means achieving unambiguous results, and this requires a sensitive and specific antibody-antigen interaction. Consequently, high quality antibodies are critical for reliable and consistent western blotting.
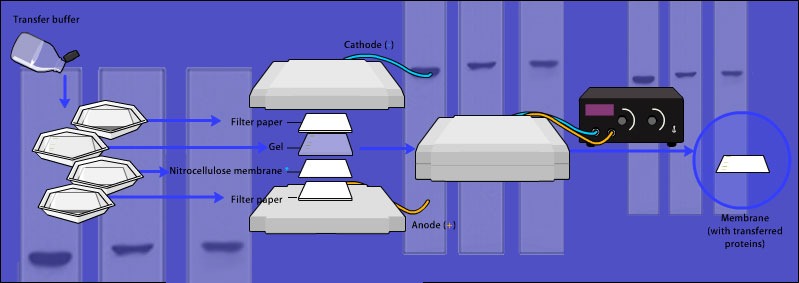
Western Blotting Process
In the basic western blotting process, polyacrylamide gel electrophoresis (PAGE) separates a mix of proteins according to their molecular weights (denaturing gels) or their 3D structures (native gels). Following electrophoresis, the proteins are transferred from the gel onto a porous membrane for easy access by blotting antibodies. Antibodies are then used to detect specific proteins. Concentration of the target protein is inferred from the intensity of the band that results from the antibody binding to a protein – either through an enzymatic reaction with your antibody or fluorescence from your antibody. Therefore, your antibody choice is critical.
Antibody-Antigen Interaction
Antibody-antigen interactions occur between the antigen-binding site (paratope) of an antibody and a small region on a protein antigen (epitope). An epitope is generally considered to be a stretch of several amino acids. An antigen normally contains multiple epitopes that can be recognized by different antibodies. Antibodies can recognize epitopes in their denatured linear, primary form (linear epitope), or their native 3D tertiary form (conformational epitope).
Antibodies that recognize linear epitopes under denaturing and reducing conditions (like in SDS-PAGE) may not detect targets whose linear epitopes are concealed in the native protein structure. On the other hand, antibodies that recognize conformational epitopes (e.g., those used in immunocytochemistry and native western blots) may lose binding affinity once target proteins are denatured.
Polyclonal Versus Monoclonal Antibodies
The antibodies commonly used in western blotting fall into two main categories: polyclonal and monoclonal antibodies.
Polyclonal Antibodies
Polyclonal antibodies are generated by immunizing laboratory animals (e.g., rabbit) with antigens of interest. The collected antibodies are often a pool of different immunoglobulin molecules recognizing different epitopes found on the same antigen. Given that one antigen can be bound by multiple antibodies, polyclonal antibodies can provide high levels of sensitivity, which may be advantageous when detecting low abundance proteins. However, because of their heterogeneous nature, polyclonal antibodies tend to give higher background and may cross-react with non-target antigens.
Monoclonal Antibodies
Monoclonal antibodies are generated by fusing an antibody-producing B cell from immunized animals with an immortalized cell line, such as a myeloma cell line . The resulting immortalized fused cells, called hybridomas, produce monospecific antibodies that recognize a single epitope on the target antigen. Thanks to their purity and specificity, monoclonal antibodies are known for lower background signals and cross-reactivity than their polyclonal counterparts.
What Are the Common Problems with Western Blots?
1. The Absence of Target Protein Bands
- Incompatibility between primary and secondary antibodies
The secondary (or detection) antibody should be raised against the species in which the primary antibody is produced (e.g., a rabbit anti-mouse secondary antibody for a primary antibody raised in mice). - Primary antibody cannot bind to epitope on denatured protein samples
If the primary antibody employed is raised against a native antigen, then it may fail to detect the denatured target protein. - The primary antibody does not recognize the protein produced in the species of interest
The epitope recognized by the primary antibody might not exist in the corresponding protein of different species.
2. Protein Bands Correspond to Incorrect Molecular Weights
- Different isoforms of the proteins
Many proteins exist in different isoforms because of alternative splicing. If the primary antibody used recognizes an epitope that’s present in the different isoforms, multiple bands may appear on the membrane. A customized antibody that recognizes a distinct isoform-specific epitope will recognize only the target isoform. - Homologous proteins of different species may differ in size
Antibodies raised against a protein of one species (e.g., mouse) can often be used to detect the same protein of a different species (e.g.,rat) because the antibodies recognize consensus epitopes present in all homologous proteins. Nevertheless, when the target protein differs in size across species, the target protein may appear at an unexpected molecular weight. - Protein degradation by protease
Proteases might digest the target protein during sample preparation or storage. Making a fresh sample with sufficient protease inhibitors is advisable. - Post-translational modification or proteolytic processing
Proteins that undergo distinct post-translational modifications, such as acetylation, phosphorylation, or glycosylation, can exist in different sizes. Moreover, certain proteins (e.g., caspase or insulin) are synthesized as a large precursor polyprotein and are cleaved into the active protein form by cellular proteases. Such cleavages can result in multiple bands on a blot if the epitope recognized by the blotting antibody is present in both the precursor and active proteins. - Insufficient denaturation of the protein sample
If a protein’s tertiary or quaternary structures are not fully denatured, then migration of the protein in gel electrophoresis will not only depend on molecular weight but also on the interaction between the tertiary structure of the proteins and the gel matrix. Extending the heat denaturation period in Laemmli sample buffer can help further denature the protein and dissociate the subunits of a multimeric protein.
3. High background and multiple non-specific bands
- The primary antibody concentration is too high
Excess primary antibody can increase the background signal through non-specific binding to the membrane or non-target proteins. To overcome this, incubate the membrane in dilute primary antibody for a longer time because that encourages a slow and targeted interaction. - Cross reactivity of primary antibodies with unrelated antigens
This often happens with polyclonal antibodies because they are less specific than monoclonal antibodies. Using an antigen affinity-purified polyclonal antibody or an alternative antibody against a different region of the target protein may solve this problem. - Insufficient blocking or washing
The non-occupied spaces on the membrane may not be fully blocked by blocking agents, or the washing steps after primary and secondary antibody incubations might not be sufficient. Block for a longer time period and/or increase the number and duration of the wash steps.
What Should You Consider When Selecting a Primary or Secondary Antibody?
A. Reduced or Non-Reduced Samples
Primary antibodies that work for denatured western blots recognize linear epitopes or internal epitopes normally buried inside a native protein tertiary structure. Antibodies of this type are suitable for reduced samples only. On the other hand, if the protein of interest is in its native, functional state (e.g., in non-denatured, native western blots), then an antibody that recognizes conformational epitopes should be used.
B. Cost, Sensitivity, and Specificity
Polyclonal antibodies are easier and cheaper to make and can offer superior sensitivity over their monoclonal counterparts. One major downside of polyclonal antibodies is batch-to-batch variation in specificity which may lead to inconsistent results. In contrast, monoclonal antibodies, which are homogeneous batches of monospecific antibody molecules, offer better specificity and consistency. Therefore, they often yield cleaner, more consistent, and more reproducible results. The downside to monoclonal antibodies is that they are more time consuming and expensive to produce, and they can be less sensitive than polyclonal antibodies.
C. Method of Detection
Various detection methods exist to visualize and quantify target proteins. Secondary antibodies labeled with different signal-producing agents can accommodate your specific needs. For example, chemiluminescence detection generally has high sensitivity but only semi-quantitative results. Furthermore, it can only detect one target protein per blot. To compare two different proteins from the same sample, you must run a separate gel or strip and re-probe your blot, which presents its own complications.
In contrast, detection with enzyme-conjugated or fluorescent secondary antibodies is less sensitive but the results are more quantitative. Using secondary antibodies conjugated with spectrally distinct fluorophores offers the opportunity to detect multiple target proteins on the same blot.
Successful Western Blotting Depends on High Quality Antibodies
Western blotting is a technique based on a precise antibody-antigen interaction. A specific and sensitive antibody will allow you to identify and analyze your protein of interest with confidence in your results. Antibody quality is arguably a critical uncontrollable factor for researchers when optimizing western blot experiments. Therefore, continuous access to high quality antibodies through a reliable source is indispensable for consistent and successful western blotting results.