Mikrogen Diagnostik recomWell Campylobacter IgA ELISA Kit (6205)
$440.00
| Host | Quantity | Applications | Species Reactivity | Data Sheet | |
|---|---|---|---|---|---|
| 96 Tests | ELISA | Campylobacter jejuni, Campylobacter coli |  |
SKU: 6205
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomWell Campylobacter IgA ELISA Kit (6205)
Description Enzyme immunoassay with antigens produced by recombinant techniques for the detection of IgG and IgA antibodies against Campylobacter jejuni and Campylobacter coli
Product is intended for research use.
Product is intended for research use.
Target Human Campylobacter IgA
Species Reactivity Campylobacter jejuni, Campylobacter coli
Assay Type Indirect ELISA
Applications ELISA
Associated Products recomWell Campylobacter IgG ELISA Kit (6204)
recomLine Campylobacter IgG Lateral Strip Test Kit (6272)
recomLine Campylobacter IgA Lateral Strip Test Kit (6273)
Campylobacter jejuni IgA Control (C139A)
Campylobacter jejuni IgG Control (C139G)
Campylobacter jejuni IgM Control (C139M)
Campylobacter jejuni IgA ELISA Kit (ESR139A)
Campylobacter jejuni IgG ELISA Kit (ESR139G)
Campylobacter jejuni IgM ELISA Kit (ESR139M)
Campylobacter jejuni Antigen (BA139VS)
recomLine Campylobacter IgG Lateral Strip Test Kit (6272)
recomLine Campylobacter IgA Lateral Strip Test Kit (6273)
Campylobacter jejuni IgA Control (C139A)
Campylobacter jejuni IgG Control (C139G)
Campylobacter jejuni IgM Control (C139M)
Campylobacter jejuni IgA ELISA Kit (ESR139A)
Campylobacter jejuni IgG ELISA Kit (ESR139G)
Campylobacter jejuni IgM ELISA Kit (ESR139M)
Campylobacter jejuni Antigen (BA139VS)
Properties
Background The genus Campylobacter comprises gram-negative, spiral-shaped, microaerophilic, mesophilic to thermophilic bacteria with bipolar flagella. Human Campylobacter infections are mainly food associated intestinal infections with world-wide incidence. Contaminated and insufficient cooked foods or drinking water in tropical countries are the main sources of infection. The pathogen reservoir is mainly the intestinal tract of warm-blooded wild, domestic and pet animals. Intestinal Campylobacter infections are the second most frequent enteric bacterial infections reported in Germany after enteric Salmonelloses, whereas unreported cases not reflected in the statistics probably outnumber reported cases many times over.Campylobacter jejuni is much more frequent, accounting for over 90% of cases as compared to Campylobacter coli at approx. 9%. Besides nearly asymptomatic (clinically inapparent) courses, infected persons suffer from painful gastrointestinal symptoms with sometimes bloody diarrhoea, fever, meningism and myalgias. In rare cases sequelae like postinfectious Reactive Arthritis or Guillain-Barré-Syndrome may develop with onset a few weeks after the primary infection.recomWell Campylobacter is a quantitative in vitro test for the detection and safe identification of IgG or IgA antibodies against specific antigens of Campylobacter jejuni and Campylobacter coli. In cases of previous or persistent Campylobacter infection with primary diagnosis based on stool sample culturing, recomWell Campylobacter, as a screening test, provides for identification of specific Campylobacter antibodies for the purpose of clarifying postinfection complications.
Sample Type Serum, plasma, whole blood
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| Dilution Buffer | 125 mL |
| TMB Substrate | 12 mL |
| Stop Slution | 12 mL |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Microplate Sealing Tape | 2 Each |
| Microtiter Plate | 12 x 8 Wells |
| Positive Control | 450 uL |
| Cut-Off Control | 450 uL |
| Negative Control | 450 uL |
| Anti-Human IgA Conjugate | 500 uL |
Specificity Information
Target ID Human Campylobacter IgA
Research Areas Infectious Disease
Application Images





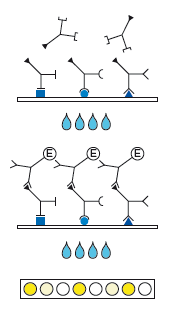
Description Test principle and procedure:
Indirect sandwich test. Recombinant antigens are bound to the solid phase.
1. 1st Incubation. Add patient samples diluted 1:101 (sample: 10 µl of serum or plasma), incubate for 1 h at 37 °C.
2. Wash 4 times
3. 2nd Incubation
4. Add peroxidase conjugated anti-human IgG or IgM antibodies (conjugate), incubate for 30 min at 37 °C.
5. Wash 4 times
6. Color reaction. Add ready-to-use TMB solution and incubate for 30 min at room temperature. Stop the substrate reaction with H3PO4 and measure the extinction at 450 nm.
Indirect sandwich test. Recombinant antigens are bound to the solid phase.
1. 1st Incubation. Add patient samples diluted 1:101 (sample: 10 µl of serum or plasma), incubate for 1 h at 37 °C.
2. Wash 4 times
3. 2nd Incubation
4. Add peroxidase conjugated anti-human IgG or IgM antibodies (conjugate), incubate for 30 min at 37 °C.
5. Wash 4 times
6. Color reaction. Add ready-to-use TMB solution and incubate for 30 min at room temperature. Stop the substrate reaction with H3PO4 and measure the extinction at 450 nm.

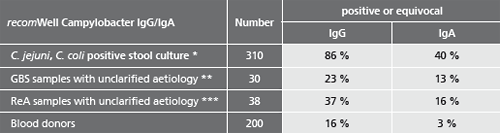
Description Test evaluation data.
Patient samples from different sources were tested to assess the performance capability of recomWell Campylobacter.
* Samples from patients with positive Campylobacter stool cultures (C. jejuni, C. coli). Blood samples were taken 0 to 81 days after the stool diagnostics, the beginning of the disease was unknown.
** Samples from patients with suspected Guillain-Barré-Syndrom (GBS) unclarified aetiology. No data on previous illnesses involving diarrhoea were known. (Testet with recomLine Campylobacter without P39).
*** Samples from patients with suspected Reactive Arthritis (ReA) unclarified aetiology. No data on previous illnesses involving diarrhoea were known.
Potentially falsifying sera of patients (n=100) infected by Yersinia enterocolitica Y. pseudotuberculosis, Helicobacter pylori, Borrelia burgdorferi, Parvovirus B19 and Toxoplasma gondii did not show a higher number of increased Campylobacter antibody titers.
Patient samples from different sources were tested to assess the performance capability of recomWell Campylobacter.
* Samples from patients with positive Campylobacter stool cultures (C. jejuni, C. coli). Blood samples were taken 0 to 81 days after the stool diagnostics, the beginning of the disease was unknown.
** Samples from patients with suspected Guillain-Barré-Syndrom (GBS) unclarified aetiology. No data on previous illnesses involving diarrhoea were known. (Testet with recomLine Campylobacter without P39).
*** Samples from patients with suspected Reactive Arthritis (ReA) unclarified aetiology. No data on previous illnesses involving diarrhoea were known.
Potentially falsifying sera of patients (n=100) infected by Yersinia enterocolitica Y. pseudotuberculosis, Helicobacter pylori, Borrelia burgdorferi, Parvovirus B19 and Toxoplasma gondii did not show a higher number of increased Campylobacter antibody titers.
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet