Mikrogen Diagnostik recomBlot Rubella IgG Immunoblot Test Kit (4902)
$660.00
SKU: 4902
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomBlot Rubella IgG Immunoblot Test Kit (4902)
Description Immunoblot test with rubella virus lysate for the detection of IgG antibodies against the Rubella virus. Differentiation test for exclusion of a Rubella primary infection.
Product is intended for research use.
Product is intended for research use.
Target Human Rubella Virus IgG
Species Reactivity Rubella
Assay Type Indirect Sandwich ELISA
Applications Immunoblot
Properties
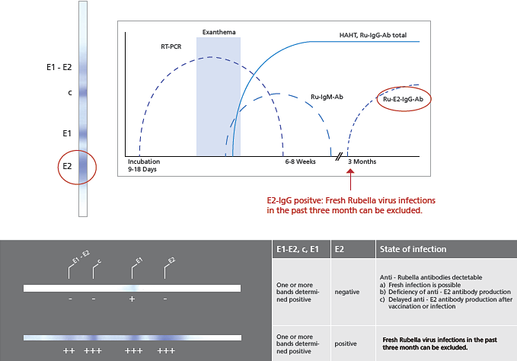
Background German measles caused by the Rubella virus typically appear during childhood and take a mild course in most cases, in many cases even proceed asymptomatically. However severe damage can occur in unborn children if the mother becomes infected with the virus shortly before pregnancy or within the first few months of pregnancy. Infections during organ differentiation within the first 12 weeks of pregnancy should be considered particularly alarming. The outcome can be spontaneous abortion, premature birth or the "Rubella syndromeâ€, the complete clinical picture for which is known as impairment of the heart, eye and inner ear (classic triad), also called Gregg's syndrome. The risk of embryopathy decreases during the further course of pregnancy. Infection past the 20th week of pregnancy is generally regarded to have no impact on the foetus.An accurate serological determination of Rubella antibody status is exceptionally important for pregnant women, if an acute infection with Rubella virus is suspected or in case of an imperfect vaccination.The recomBlot Rubella IgG uses Rubella virus lysate produced in Vero cells. All Rubella specific antigens which are important for a accurate serological determination are presented by electrophoretic separation and subsequent transfer to a nitrocellulose membrane (Western Blotting). IgG conformation antibodies against the Rubella antigen E2 appear three month after vaccination or infection at the earliest. An infection within the last three month can be serologically ruled out if the E2 band is detectable.
Sample Type Serum, plasma, whole blood
Assay Resolution See Figure
Components
| 5X Wash Buffer | 100 mL |
| TMB Substrate | 40 mL |
| Incubation Tray | 2 Each |
| IControl Strip | 1 Each |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Test Strips | 2 Vials x 10 Each |
| Weak Positive Control | 120 uL |
| Negative Control | 100 uL |
| Anti-Human IgG Conjugate | 500 uL |
Specificity Information
Target ID Human Rubella Virus IgG
Research Areas Infectious Disease
Application Images






Description Strip layout.

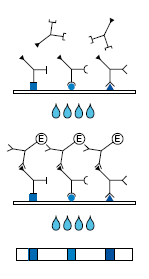
Description Test Procedure and Principle:
1. 1st Incubation. A test strip loaded with Rubella antigens is incubated with diluted serum or plasma in a dish for 10 - 16 hours.
2. Wash 4 times
3. 2nd Incubation
4. Peroxidase conjugated anti-human antibodies (IgG specific) are added. Incubate for 60 minutes.
5. Wash 4 times
6. Color reaction
7. 10 - 15 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies.
1. 1st Incubation. A test strip loaded with Rubella antigens is incubated with diluted serum or plasma in a dish for 10 - 16 hours.
2. Wash 4 times
3. 2nd Incubation
4. Peroxidase conjugated anti-human antibodies (IgG specific) are added. Incubate for 60 minutes.
5. Wash 4 times
6. Color reaction
7. 10 - 15 minutes after addition of the coloring solution, insoluble colored bands develop at the sites on the test strips occupied by antibodies.

Description Rubell markers - (according to B. Pustowoit)
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet